Cell Science at a Glance.
Yvonne Collins, Edward T. Chouchani, Andrew M. James, Katja E. Menger, Helena M. Cochemé and Michael P. Murphy* .
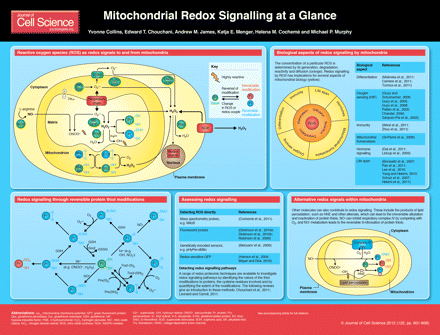
Redox signalling occurs when a biological system alters in response to a change in the level of a particular reactive oxygen species (ROS) or the shift in redox state of a responsive group such as a dithiol–disulphide couple (D'Autreaux and Toledano, 2007; Finkel, 2011; Fourquet et al., 2008; Janssen-Heininger et al., 2008; Rhee, 2006). Although ROS are best known as damaging agents in pathology, a more nuanced view has developed.
It is now clear that some ROS, such as hydrogen peroxide (H2O2), can act as messengers both in the extracellular environment and within cells (D'Autreaux and Toledano, 2007; Fourquet et al., 2008; Janssen-Heininger et al., 2008; Rhee, 2006). Mitochondria seem to be an important redox signalling node, partly because of the flux of the ROS superoxide (O2·−) generated by the respiratory chain and other core metabolic machineries within mitochondria (Balaban et al., 2005; Finkel, 2011; Murphy, 2009a). In addition, the mitochondrial matrix is central to metabolism, as oxidative phosphorylation, the citric acid cycle, fatty acid oxidation, the urea cycle and the biosynthesis of iron sulphur centres and haem take place there. Furthermore, mitochondria have key roles in apoptosis, calcium homeostasis and oxygen sensing (Duchen, 2004; Murphy, 2009a; Murphy, 2009b). Consequently, mitochondria are at the core of many biological processes, and redox signals to and from this organelle help to integrate mitochondrial function with that of the cell and organism. In this Cell Science at a Glance article we outline how mitochondrial redox signals are produced and modulated, the mechanisms by which redox signals can alter mitochondrial function and the experimental procedures available to assess this.
Production and modulation of redox signals to and from mitochondria
The initial ROS formed within mitochondria is O2·−, which is generated by the respiratory chain and other enzymatic components within the mitochondrion (Finkel, 2011; Murphy, 2009a). Mitochondrial O2·− generation provides an indication of functional status because its production is altered by many cellular factors. These include the membrane potential, the reduction state of electron carriers and post-translational modification or damage to the respiratory chain (Murphy, 2009a). However, O2·− itself is not the main ROS signal within mitochondria because it is mostly converted to H2O2 by manganese superoxide dismutase (MnSOD), which reacts very rapidly with O2·− and is present at a high concentration within the matrix (Balaban et al., 2005; Chance et al., 1979; Finkel, 2005; Murphy, 2009a). As H2O2 can pass easily through mitochondrial membranes, it can act as a redox signal from mitochondria to the rest of the cell and vice versa (Balaban et al., 2005; D'Autreaux and Toledano, 2007; Droge, 2002; Fourquet et al., 2008; Janssen-Heininger et al., 2008; Murphy, 2009a). Respiratory complex III can also release O2·− into the intermembrane space (St-Pierre et al., 2002; Muller et al., 2004; Han et al., 2001). The intermembrane space enzyme p66Shc (the 66 kDa isoform of the growth factor adapter Shc) can also generate O2·−, which can regulate apoptotic cell death (Giorgio et al., 2005). The O2·− can diffuse from the intermembrane space to the cytosol or be converted to H2O2 by an intermembrane space Cu,Zn-SOD (Okado-Matsumoto and Fridovich, 2001). The Mia40p and Erv1p system of the intermembrane space, which inserts disulphide bonds into intermembrane space proteins during import, also generates H2O2 (Koehler et al., 2006), but the potential of this for redox signalling is unclear. Full article: link above "Mitochondrial redox signalling at a glance - Full article!